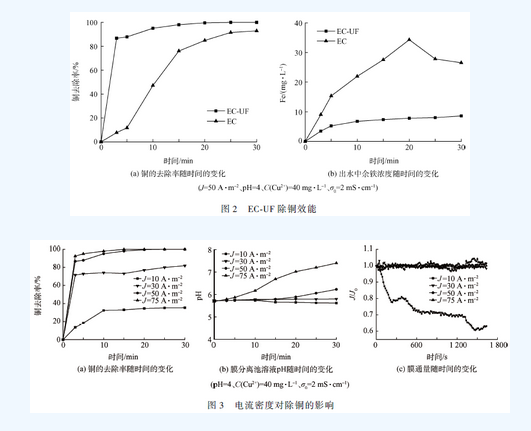
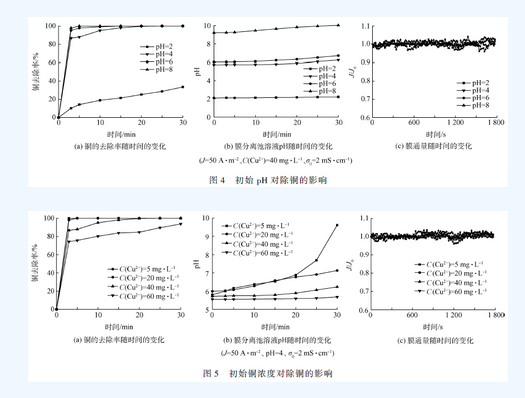
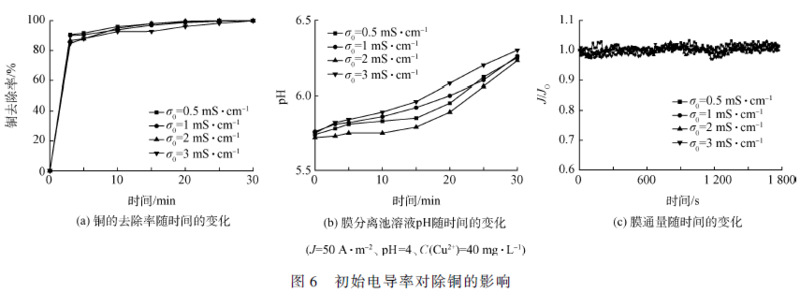
Smm network: Copper is a very common heavy metal in industrial wastewater. If it is directly discharged into the environment without proper treatment, it will seriously threaten the health of the aquatic ecosystem and endanger human health through the food chain. At present, the methods for treating copper-containing wastewater mainly include chemical flocculation, ion exchange, adsorption, and electrocoagulation. As an efficient water treatment technology, electroflocculation has the advantages of simple operation, small amount of mud production, avoidance of using chemicals, easy automation and equipment control. However, as the pollution situation continues to intensify and the limits of heavy metals in water quality standards become more stringent, electroflocculation technology often requires other technologies to meet water quality discharge standards. Membrane filtration technology has a good treatment effect on suspended particles, organic compounds and inorganic pollutants such as heavy metals, but membrane fouling restricts its popularization and application. Electroflocculation as a pretreatment unit for membrane separation can effectively reduce membrane fouling. Therefore, the combination of electroflocculation and membrane separation technology can not only shorten the water treatment process, improve the separation efficiency of pollutants, but also effectively reduce membrane fouling, increase membrane flux, and have good synergistic effects. In this study, electro-flocculation-ultrafiltration (ec-uf) combination technology was used to treat industrial copper-containing wastewater. The effects of current density j, initial pH, initial copper concentration and initial conductivity on copper removal were investigated. Electrocoagulation and ultrafiltration were analyzed. Combine the mechanism of copper removal and explore the membrane fouling situation, which provides a technical basis for practical application in the ec-uf copper removal process. 1 Materials and methods 1. 1 experimental materials Plate and membrane material: In the experiment, the anode and cathode were made of iron plate, and the plate size was 115 mm 65 mm 2 mm (effective area 68 cm2). The hollow fiber ultrafiltration membrane module was supplied by Tianjin Membrane Group. The ultra-filtration membrane material is polyvinylidene fluoride (pvdf), and the membrane pore size is 0.03 m. Simulated experimental wastewater: Add cuo4 ̇ 5h2 o to deionized water to maintain the initial concentration of cu2 + at c(cu2 + ) = 40 mg ̇l - 1 . The solution ph was adjusted with 1 mol ̇l -1 of naoh and hcl. To the solution was added 0.5 mmol of ̇l - 1 of nahco3 as a buffer material and adjusted to a conductivity of 2 ms ̇ cm -1 with anhydrous na2 so4. The drugs used in the experiments were of analytical grade. 1. 2 experimental device The experimental setup is shown in Figure 1. In this experiment, a self-made plexiglass tank (effective volume of 450 ml) was used as the reactor, and the plate spacing was 20 mm. In the experiment, the dh1765-1 type program-controlled DC stabilized current source (35 v, 3a) was used; the solution was stirred by a magnetic stirrer to make the electrolyte evenly dispersed in the reactor. The experimental simulated wastewater enters the electro-flocculation tank through the pump and then enters the membrane separation tank. A part of the water is separated and flows out through the membrane, and another part of the water that is gradually retained in the membrane separation tank is returned to the original pool by the pump due to the decrease of the membrane flux. 1. 3 analysis methods The pH was measured by a pH meter (720, thermo orion, usa). The total copper and total iron concentrations were determined by inductively coupled plasma-atomic emission spectroscopy (icp-oes optima-2000, perkinelmer, usa). Conductivity was determined by conductivity. Mettler toledo (s230), the experiment uses the relative flux j / j0 to characterize the degree of membrane fouling, tmp and electronic scale readings are recorded using the relevant sensors and corresponding data acquisition software. In order to prevent the electric field from producing errors in the ph meter measurement, the ph of the electro-flocculation tank is indirectly reflected by measuring the ph of the membrane separation tank, and the ph of the solution in the membrane separation tank is monitored in real time, since the solution first enters the membrane separation tank through the electro-flocculation tank. Therefore, the initial pH of the membrane separation tank solution is higher than the initial pH of the raw water. 2 results and discussion 2. 1ec-uf copper removal efficiency study The copper removal effect of ec-uf and ec was compared and the results are shown in Fig. 2. It can be seen from Fig. 2(a) that the efficiency of copper removal by ec-uf can reach about 85% after 5 minutes of filtration. With the progress of electrolysis, the removal rate of copper can reach 99.6% after 20 minutes. The effluent water quality is stable, and the residual iron concentration of the filtrate is less than 10 mg ̇l -1 , which can reach the water quality standard of sewage discharged into the city. The removal rate of copper by ec can reach 92.9% at 30 min, and it can be seen from Fig. 2(a) that the iron concentration of the ec process effluent reaches 26 mg ̇l -1 , which exceeds the water quality of sewage discharged into the city. standard. The ultrafiltration membrane effectively improves the efficiency of electro-flocculation and copper removal. The ec-uf process is superior to the ec process in terms of copper removal rate and effluent quality. 2. 2 The effect of current density on copper removal The current densities were selected as 10, 30, 50 and 75 a ̇m - 2 respectively. The effect of current density on copper removal was investigated. The results are shown in Fig. 3. It can be seen from Fig. 3(a) that the greater the current density, the faster the copper removal rate. This can be explained by the fact that according to faraday's law, the higher the current density, the more iron ions are oxidized from the anode, which is equivalent to increasing the dosage of flocculant per unit time. It can be seen from Fig. 3(b) that the ph of the membrane separation cell solution increases with the increase of the current density, indicating that the current density increases and the cathode also produces more hydroxide. Therefore, the increase of current density, one is to promote the formation of iron hydroxide and other hydroxy iron complexes, so as to play the role of adsorption bridge and net trapping sweep to remove copper [11]; the second is copper ions and hydrogen The root combines to form copper hydroxide for the purpose of removing copper. It can be seen from Fig. 3(c) that electroflocculation as a pretreatment of membrane filtration can well retard membrane fouling. The attenuation of the membrane flux decreases with increasing current density. During the reaction time of 30 min, when the current density is greater than 30 a ̇m - 2 , the flux of the membrane is hardly attenuated. This is because the iron ions electrolyzed from the anode in the reactor increase with the increase of the current density, and the ec-uf process is a continuous flow in series, which provides a certain time for the growth of the flocs, which makes the membrane filtration. The floc size in the reactor is relatively large, and a relatively loose filter cake layer is formed on the surface of the membrane, thereby reducing membrane fouling. Although the higher the current density, the higher the copper treatment rate, the lower the decay rate of the membrane flux, but the optimum current density is chosen to be j = 50 a ̇m - 2 in consideration of the energy consumption problem. 2. 3 initial ph effect on copper removal The initial phs were selected as 2, 4, 6, and 8, respectively. The effect of the initial ph on copper removal was examined. The results are shown in Fig. 4. It can be seen from Fig. 4(a) that as the initial ph increases, the copper removal rate also increases. Because under acidic conditions, the process of oxidation of ferrous iron to ferric iron will be weakened, and in the case of lower ph, the hydroxy iron complex is difficult to form, and the dissolved iron salt is difficult to function effectively, so copper The removal rate is reduced. Under alkaline conditions, ferrous iron is more easily oxidized to ferric iron to form iron hydroxide and more complex polymers, and copper ions are also easily combined with hydroxide to form copper hydroxide, so that good removal can be achieved. . It can be seen from Fig. 4(b) that under the condition of initial ph = 2, since a large amount of hydrogen ions exist in the raw water, the ph variation of the membrane separation tank solution is relatively small as the reaction time. Under acidic conditions, the hydroxy iron complex and copper hydroxide are difficult to form. Therefore, cu2 + undergoes redox reaction on the iron plate to form a layer of red copper. The removal of copper is completely dependent on electrodeposition, so the removal rate is not very high. high. As can be seen from Fig. 4(c), the membrane flux hardly attenuates under the conditions of initial ph of 2, 4, 6, and 8. At the initial ph = 2, the membrane flux did not decay because there was not a large amount of floc formation and the ultrafiltration membrane did not have a trapping effect on the ion-formed particles. Under the conditions of initial ph of 4, 6 and 8, due to the relatively large particle size of the flocs, a relatively loose filter cake layer is formed on the membrane, thereby reducing membrane fouling. 2. 4 The effect of initial copper concentration on copper removal The initial c(cu2 + ) = 5, 20, 40 and 60 mg ̇l - 1 were selected to investigate the effect of initial c(cu2 + ) on copper removal. The results are shown in Fig. 5. 5以上。 When the initial copper concentration of 60 mg ̇l -1, the treatment efficiency reached 93.4% at 30 min. When the initial copper concentration is less than or equal to 40 mg ̇l -1 , the copper treatment rate is almost 100% at 20 min. It can be seen from Fig. 5(b) that the ph of the membrane separation tank solution increases slowly with the increase of the initial copper concentration without changing the current density, indicating that cu2 + is generated at the cathode and hydroxide in the ec-uf process. Copper hydroxide is also a way to remove copper. It can be seen from Fig. 5(c) that the initial copper concentration has no effect on the membrane flux due to the action of the filter cake layer on the membrane surface within 30 min of the reaction time. In summary, when the initial c(cu2 + )40 mg ̇l -1 , the ec-uf process runs for 20 min, almost all the copper in the wastewater can be removed and the membrane fouling is effectively alleviated. 2. The effect of initial conductivity of solution on copper removal The initial solution = 0, 5, 1, 2 and 3 ms ̇ cm - 1 were selected to investigate the effect of initial conductivity of the solution on copper removal. The results are shown in Fig. 6. As can be seen from Fig. 6, the copper treatment rate, the ph of the membrane separation cell solution, and the attenuation of the membrane flux hardly change with the change of the initial conductivity of the solution. Although the conductivity is too large to affect the quality of the effluent, an appropriate increase in conductivity can ensure that the power consumption is effectively reduced while the current density is constant. Taking into account, the initial conductivity of the solution was chosen to be 2 ms ̇ cm - 1 . 3 conclusions 1) ec-uf is an effective technique for removing copper ions from wastewater. Its copper removal efficiency is about 15% higher than ec, and membrane fouling is effectively alleviated. 2) Increasing the current density and the initial pH of the solution can increase the removal rate of copper ions. Appropriately increasing the initial conductivity of the solution not only does not affect the experimental results, but also effectively reduces the power consumption. 3) The process conditions are: j = 50 a ̇m - 2 , initial ph = 4 ~ 8, initial c (cu2 + ) 40 mg ̇l - 1 , initial = 2 ms ̇ cm - 1 , electrolysis 20 min, remaining The copper ion concentration was 0.14 mg ̇l -1 , and the removal rate reached 99.6%, which reached the water discharge standard of sewage discharged into the city, and the membrane flux remained at the initial level within 20 min, with almost no decrease. Welded Wire Mesh,Metal Wire Mesh,Welded Wire Fencing,Industrial Metal Welded Wire Shenzhou City Hongda Hardware Products Co.,Ltd , https://www.hdpvcwire.com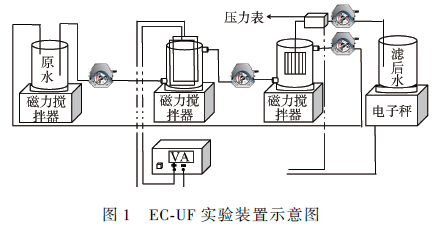
/*kangxianyue 250*250 was created on 2017/3/29*/ var cpro_id = "u2939694";