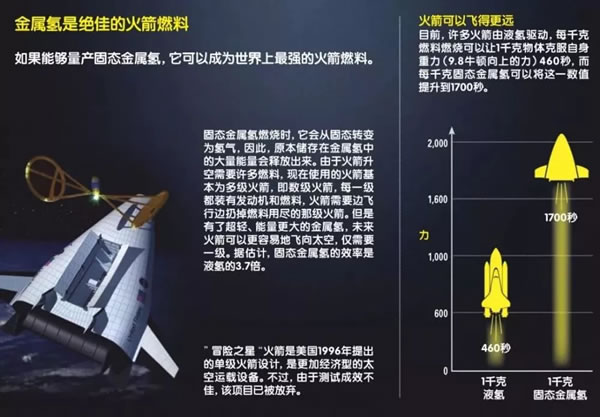
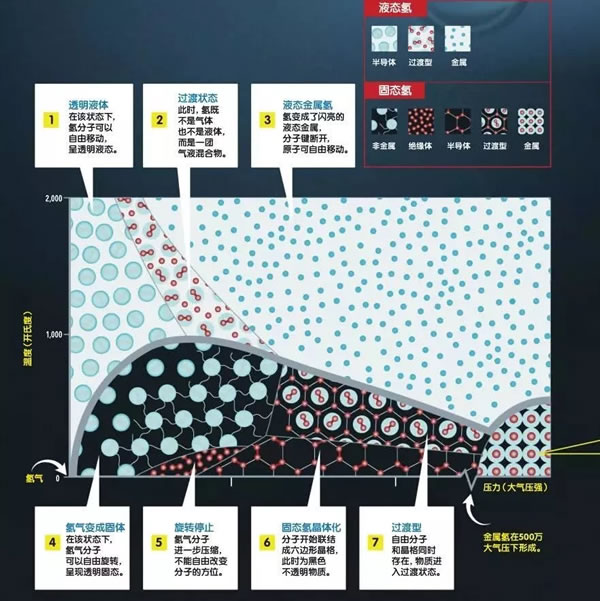
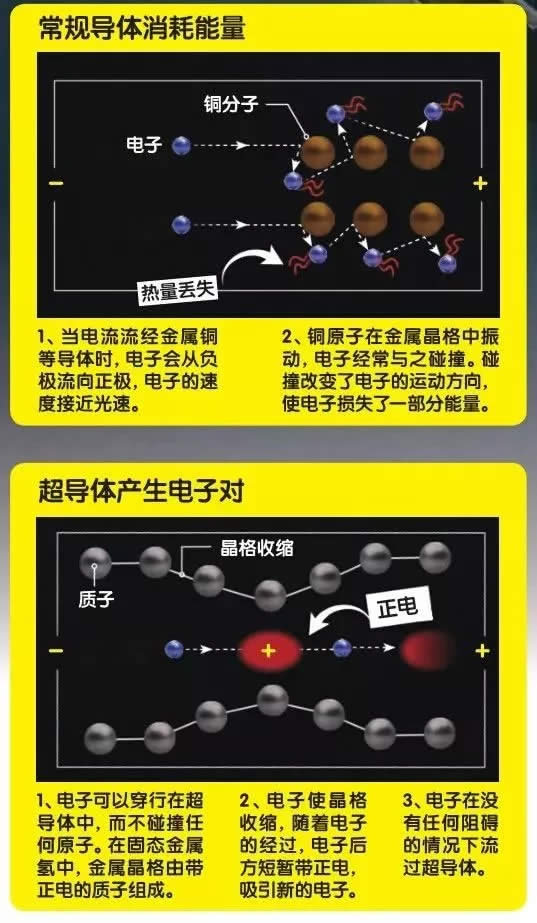
In the National Ignition Device (NIF) laboratory, the hydrogen sample in the small test chamber is under tremendous pressure. The pressure is so great that the hydrogen gas changes state and becomes a liquid. However, this is just the beginning, as the pressure gradually becomes greater, the small droplets also undergo a series of changes. Hydrogen is the most abundant element in the universe. The main component of gas planets such as Jupiter and Saturn is hydrogen, but inside the planet, the form of hydrogen is not gaseous, but metal hydrogen. Therefore, the small changes in hydrogen droplets can tell us a lot about planets such as Jupiter and Saturn. At the same time, solid metal hydrogen will be a magical future material. It can be used as a fuel to make the spacecraft fly further; it can also be used as a superconductor to allow current to flow without resistance. Multiple states of simple matter There are three isotopes of hydrogen: protium, deuterium, and tritium. 99.985% of hydrogen in nature is protium. It has the simplest atomic structure in the world, with a proton surrounded by an electron. In addition, the deuterium nucleus is composed of a proton and a neutron, and the content in nature is generally about one-seven thousandth of all hydrogen elements. The tritium nucleus consists of one proton and two neutrons. It is radioactive and rarely exists in nature. In the laboratory, you can also artificially synthesize four other isotopes of hydrogen: hydrogen 4, hydrogen 5, hydrogen 6, and hydrogen 7. In our natural world, hydrogen mainly exists in the form of hydrogen, which is composed of two atoms joined together to form a molecule. However, as the pressure and temperature change, the density and arrangement of atoms will change, so hydrogen actually has a variety of states. Under normal pressure, when the temperature drops to -252.88 ° C, hydrogen will become a liquid, and when it drops to -259.125 ° C, hydrogen will become a solid. But at high temperature, as the pressure increases, the hydrogen gas will first become clear liquid hydrogen, at which time the molecules can flow freely; then it becomes an opaque liquid hydrogen, at this time there are both molecules and atoms in the liquid; the last molecule The bond is completely broken and becomes a liquid metal hydrogen composed of atoms. Finally, as the pressure increases, the hydrogen nuclei are aligned together, and the electrons can move freely. At this time, they are in the state of solid metal hydrogen. At present, the researchers' focus is on liquid and solid metal hydrogen. In August 2018, researchers at the NIF laboratory in the United States found an accurate method to convert deuterium to metal form under conditions above 726.85 ° C and 2 million atmospheric pressure. Although the liquid metal hydrogen in the laboratory is very unstable, it can allow researchers to understand the properties of some liquid metal hydrogen, such as whether liquid metal hydrogen is a superfluid liquid. If the liquid metal hydrogen is a superfluid liquid, it will play a vital role in understanding the movement mode of the gas planet and the external magnetic field. Astronomers speculate that there are oceans of liquid metals inside gas planets such as Jupiter and Saturn. 80% of these giant planets are made of liquid metal hydrogen, not just gas. In superfluid liquids, the flow of particles does not encounter any resistance. Once the liquid starts to move, it can move indefinitely, which is probably the reason for Jupiter's strong magnetic field. Looking for solid metal hydrogen Although the road to manufacturing solid metal hydrogen has a long way to go, once successful, it will bring a major leap in the field of metal hydrogen research. The study of solid metal hydrogen began in 1935, when American physicists Eugene Wigner and Hillard Huntington predicted that under very high pressure, hydrogen could be transformed into a solid substance with metallic properties, and the atomic structure should be compact 10 times. At the same time, once a solid substance is produced, it can maintain its state and metallic properties even under normal pressure, just like diamonds. Diamonds are formed from carbon under high pressure and high temperature inside the earth. When diamonds are mined from the ground, it can still maintain a compact atomic structure instead of expanding into graphite. The scientist's closest success was in 2017. At a very low temperature, scientists at Harvard University in the United States used a diamond countertop anvil (consisting of two diamonds and a gasket, the sample is placed in the center of the diamond and the gasket). The hydrogen sample exerted 4.95 million times the atmospheric pressure (the core pressure is about 360 times the atmospheric pressure). As the pressure gradually increased, hydrogen turned from a non-conductive transparent insulator into a black semiconductor, and finally into a shiny and shiny metal solid. At this time, the force between the hydrogen atoms is converted into metal bonds, the electrons outside the hydrogen nucleus are freed from the shackles, and the nucleus shares a group of electrons. Regrettably, the only metal hydrogen in the world disappeared after only a month or so. After the solid metal hydrogen is generated, it is kept in the diamond anvil. Before the sample is sent to the Argonne National Laboratory, the researchers want to use the laser to test the pressure for the last time. As a result, the diamond is broken, and the solid metal hydrogen sample is like this. It was found in the debris doped with diamonds. Now scientists have been improving and repeating the experiment. Future technological revolution Today, many rockets are driven by liquid hydrogen (prepared by liquefaction at a standard atmospheric pressure and a temperature of -253 ° C). If we use solid metal hydrogen as a fuel, when it burns, it will start from the solid state. Converted into hydrogen, at this time, the energy when the solid metal hydrogen becomes hydrogen will be released and then burned. Therefore, this super metal can produce more energy than liquid hydrogen fuel. Researchers predict that solid metal hydrogen is 3.7 times more efficient than liquid hydrogen. Modern rockets often have to travel in space for a long period of time, so they need a lot of fuel. For this, they need to be equipped with huge fuel tanks. Rockets are often very large. Once the solid metal hydrogen is successfully developed, future rockets can become lighter and more efficient, greatly reducing the difficulty and cost of space navigation. In addition to being a fuel, another important application of solid metal hydrogen is as a superconductor. Current superconductors must be cooled to -269 ° C with liquid nitrogen to maintain extremely low resistivity, which is expensive and requires energy. However, according to theoretical predictions, solid metal hydrogen is a superconductor at room temperature with a resistivity of zero. This may pave the way for a technological revolution. We can store electricity from green energy in a large superconducting coil composed of solid metal hydrogen. Since the resistivity is completely zero, the current flowing in it will not consume any energy. Can continue to flow. Although the road to developing solid metal hydrogen and achieving commercial mass production still seems to be a long way, the beginning of each new technology is difficult. With the breakthrough in this field in recent years, I believe we will soon see hydrogen metal blocks at room temperature.
We diviided the led light according to the using eviiroment. Most of our product is the commercial LED Lighting
Commercial lighting is a term used to describe lighting that is used in commercial spaces, including auto dealerships, distribution centers, churches, factories, offices, and warehouses. Unlike residential lighting, commercial lighting is made to withstand more abuse and has a longer lifespan.
While the focus of residential lighting is often on aesthetics, commercial lighting is task orientated. Commercial lighting systems are designed based on what the application is. For example, in an office-type setting, you may see task lighting, which illuminates specific areas where employees need concentrated light to be able to perform their jobs.
Magnetic Linear Light,Mangnetic Linear Tracklight,Magnetic Lighting Track,Recessed Magnetic Track Light Jiangmen Dilin Lighting High-Tech Co., Ltd. , https://www.dilinlight.com